
In order to enhance your pharmaceutical or cosmetic dossiers, we can offer to carry out your cutaneous absorption studies according to the OECD 428 guidelines and in compliance with Good Laboratory Practice.
BIO-EC Laboratory has high performance Microette Plus (Hanson Research) systems enabling the parallel use of 12 Franz cells.
They have many advantages:
Whether raw materials, formulations under development or finished products are involved and given the broad action spectrum of this system, BIO-EC Laboratory daws up a specific protocol for each request.
Based on the nature of the product to be tested it can be assayed in the various compartments of the skin (stratum corneum, epidermis, dermis) and in the receiving medium.

In order to best characterise the penetration of the product tested through the various layers of the skin, the assays are carried out using the appropriate analytical techniques (HPLC, GPC-MS, ICP, Raman, etc.).
These assays are carried out in compliance with the validated and specific analysis methods for the target molecule.
Within the context of GLP studies, these analytical validations can be carried out in compliance with the EMEA guidance: Guidelines on bioanalytical method validation.


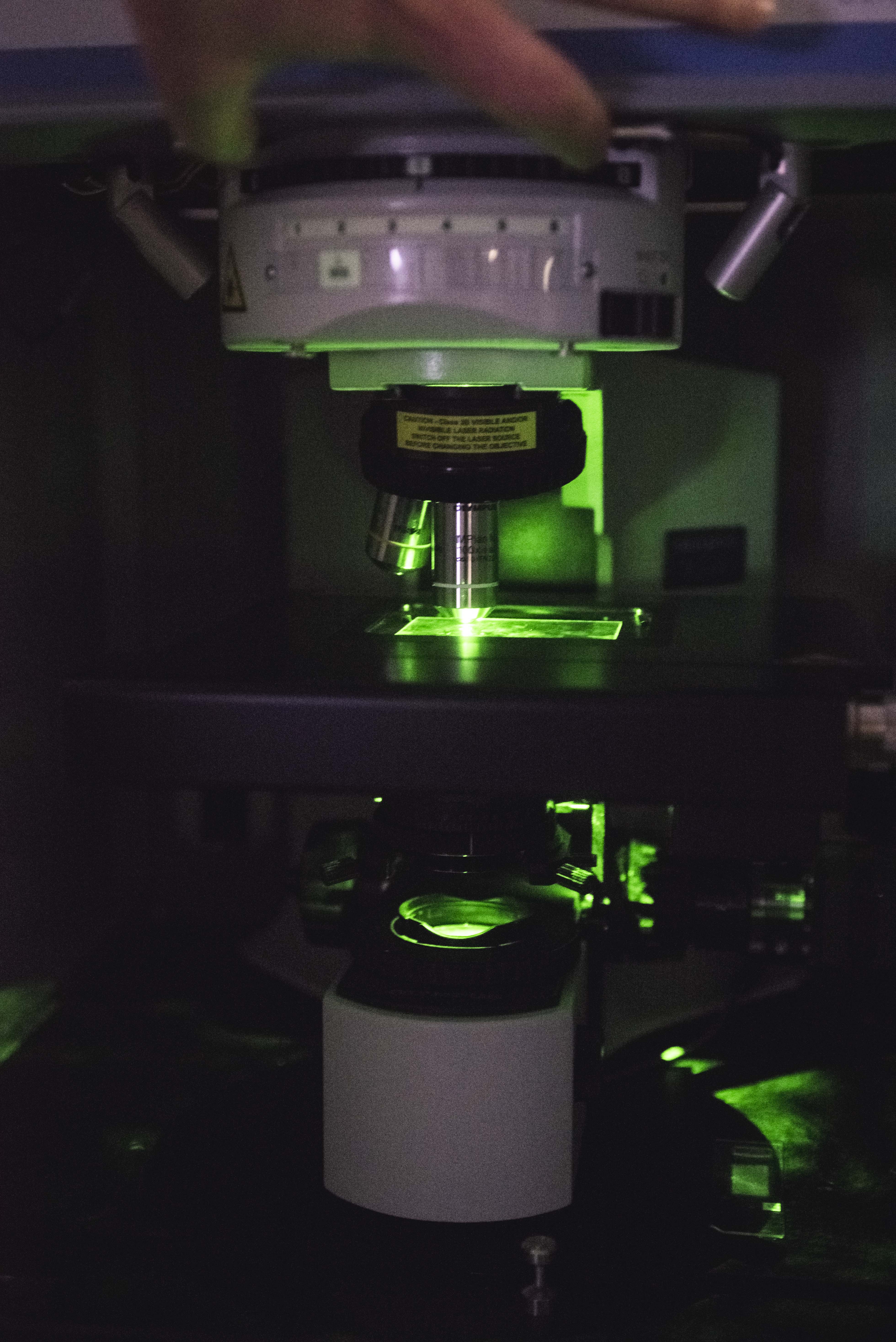
This non-invasive, sensitive and quick method provides qualitative and semi-quantitative information (on a molecular, spatial and temporal level). The Raman data enable spectral images to be constructed associated with the biological system.
Raman spectroscopy enables carrying out ex vivo studies on frozen slices and in vivo studies on volunteers.
Our laboratory is equipped with a Horiba Jobin Yvon spectroscope (Raman XploRA confocal microscope) which is fully automated and fitted with three lasers and the LabSpec software.
A study using Raman spectroscopy enables you:
to evaluate the penetration kinetics and the tissue distribution of your product (ex vivo on frozen slices). This technique gives access to data of prime importance both for the rational development of a formulation and for the constitution of the cosmetic safety file.
to evaluate the modifications caused by your product on the skin in terms of various parameters (hydration, barrier function of the skin, etc.).
to evaluate the distribution of cutaneous lipids, proteins, keratins, nucleic acids, carotenoids and components of the NMF such as serine, proline, urea and PCA.
Many technical advantages are linked to the use of this method for cosmetic objectivation studies:

Entrez votre adresse email pour vous désabonner.
This message only appears when you first log